By Francoise Collanges
My MA project involves making a copy of the working elements of a mid-19th century electro-mechanical clock in order to see what the process of making and the object could bring to conservation as a research tool.
One of the first questions was how was the clock originally powered, and would it be possible to reproduce this today? Research revealed it was a type of cell invented in 1836 by John Frederick Daniell, who gave it his name. Twenty years after the invention, his system was still widely used for providing electrical power, remaining within some specialized fields well into the twentieth century.
The next questions were how was the cell constructed, would it produce a constant enough current for reliable operation of the clock, and would the voltage be similar to a contemporary cell? All these answers have an impact on understanding the original object. With the support and collaboration of two other West Dean Clocks Programme students, Jonathan Butt and Kenneth Cobb, materials were gathered.
A first attempt at making a cell before Easter 2012 showed some success. It used chemicals (zinc and copper sulphates) described in later formulas of Daniell's, and although the proportions were determined empirically, this preliminary trial ran for several days.

In order to re-create a cell closer to the original as described by Daniell, the following was needed :
- A porous vase. This was provided by Alison Sandeman, West Dean pottery tutor, who shaped and fired the biscuit porcelain at 800° C.
- A zinc anode: luckily, West Dean is close enough to the sea to find a company specialized in anodes for boat protection.
- A copper pipe. This was shaped from a sheet material provided by the metals workshop.
- Sulphuric acid solution (11% in deionized water)
- Copper sulphate (225gr at least)
- A glass Kilner jar.
To monitor the results, the data was taken every 15 minutes by an electronic device (thanks again to Kenneth Cobb) and software logging results direct into Excel.
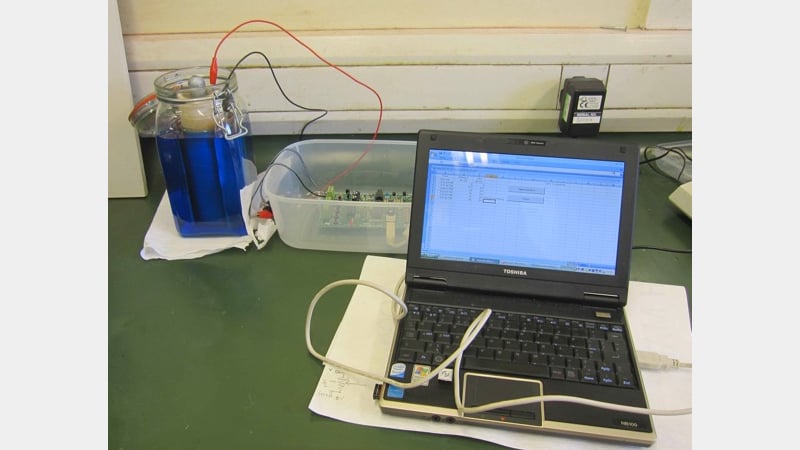
The solution inside the vase started to fume and bubble straight away and produced current at its maximum voltage. By placing a resistance simulating the average resistance which the original clock should have had in the circuit (22 ohms), 1 volt was obtained. Without that extra resistance, the voltage was slightly higher.
After some hours, the bubbles settled but the voltage remains amazingly constant. Small drops of currant appeared after 20 hours but were short. The solution level within the vase had dropped to almost half. Copper crystals are starting to form on the surface and thick crystals are visible at the bottom of the glass jar. It was the collapse of the porous vase which finally ended the test.
So we made and tested a cell as it may have been made in 1836. Testing and research far from finished, and the results need to be more accurately analysed. However, it can already be said that:
- This clock was powered with a voltage inferior to the smallest one available nowadays (1.5 volt battery).
- It didn't draw a huge amount of current from the cell, so the life length of the cell should have been of several days at least, even with the first generation of Daniell's cell (and probably longer with the one produced in 1855).
The current produced was very constant at the beginning, with drops of several minutes when the cell was starting to degrade.

Enough to imagine the way that early electric clock as a system was working in a slightly different way from how we generally perceive them today.


